- Home
- INNOVATION
- Safety Assurance
- Shiseido Safety Assurance System
Shiseido Safety Assurance System
Elements of Shiseido Safety Assurance System
We have been committed to conducting research on safety assurance for many years as well as alternatives to animal experiments for decades, and the Shiseido Safety Assurance System encompasses the collective findings of this research.
The system comprises five elements in total: three steps (“safety assurance by examining the existing toxicological data,” “safety assurance by alternative testing methods,” and “final safety assurance by human testing”), an additional approach to the second step (“safety assurance by alternative testing methods”), and “response to consumer requests.” For each element, an appropriate series of specific tests are performed to ensure its safety.
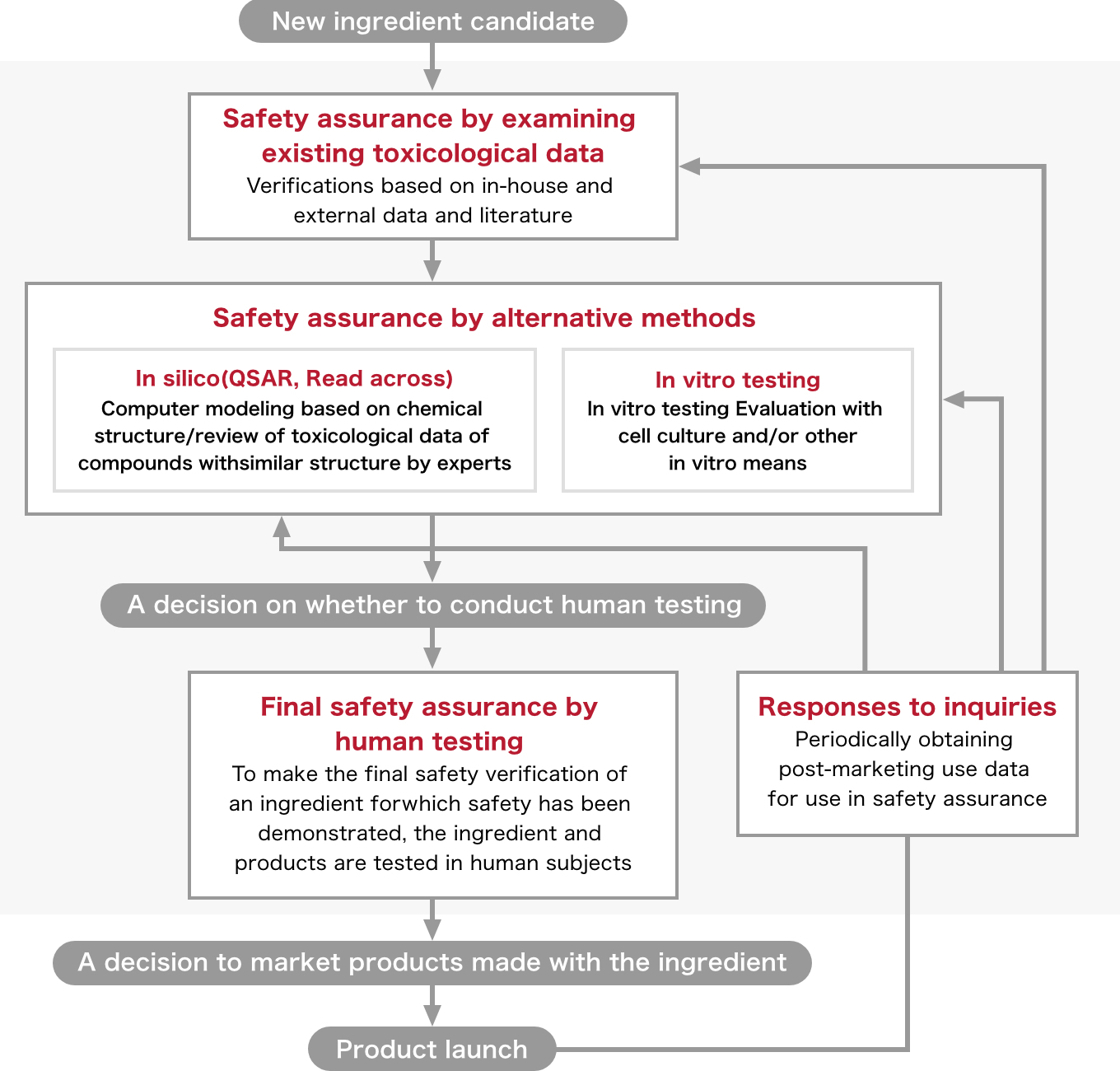
Safety assurance by examining existing toxicological data
Shiseido has accumulated invaluable experience and data through its safety evaluation research conducted for many years.
We created a database to make accumulated knowledge and data easily accessible. All data stored in the database including post-marketing data is constantly updated. In addition to in-house data, we use publicly available data such as research papers and public reports/databases that have been confirmed as reliable.
As safety data on chemical substances is constantly updated around the world, we spend a considerable amount of time and energy to obtain accurate data. Under such circumstances, researchers in charge of safety assurance continuously work to improve their evaluation skills by participating in global-level safety evaluation events held by the industry or academic societies in order to conduct appropriate safety assurance tests by examining existing toxicological data.
Safety assurance by alternative testing methods
In silico testing (quantitative structure-activity relationship [QSAR] models, read across)
Assessments are conducted using mathematical models or knowledge-based models.
In the case of known ingredients similar to if not necessarily identical to a new ingredient, that is, if there is safety data indicating similar chemical structure and/or biological reactions, then that safety data may be utilized for safety assurance tests. Data used for safety assurance is validated for each evaluated item by an expert in the relevant field.
In vitro testing
The safety of ingredients can be evaluated through in vitro studies conducted with experimental models such as cell cultures. Depending on the purpose, a variety of methods are available. In some cases, a series of tests can be applied to assess the safety of an ingredient.
Final safety assurance by human testing
Regarding the safety of new ingredients assured by existing toxicological data and by alternative testing methods, a final safety assessment is conducted via human testing. Before moving on to this step, the Human Testing Ethics Review Council carefully discusses whether human testing should be allowed or not based on the results of the verifications and tests that were performed until that point in time.
Additional safety assessments on some final products may be conducted even after they have demonstrated safety at the ingredient level. In Shiseido, final safety assessments by human testing are implemented in accordance with precisely designed protocol, and tests are mainly conducted as follows:
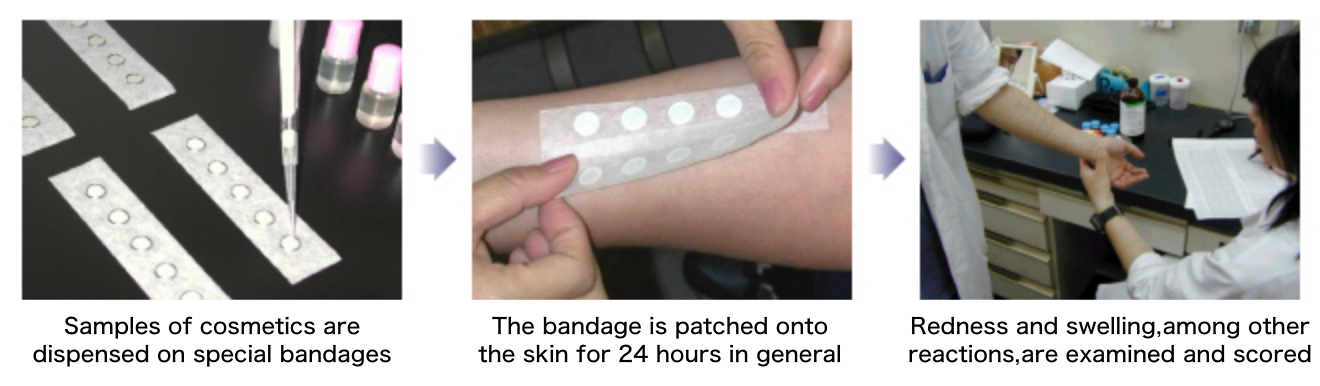
I. Patch test
The patch test aims to assess skin irritation potential of the test ingredient or product. A small amount of the test ingredient or product smeared onto a patch is applied to the inside of the forearm or to the back of the subject, and left for 24 hours or 48 hours. After the patch is removed, a highly experienced specialist examines the skin where the patch was applied, and checks for allergic reactions such as redness and swelling to assess the irritative effects of the ingredient or product. (Patch test results may be assessed by a dermatologist if necessary.)
II. Sting test
The sting test aims to assess uncomfortable sensory irritations (stinging) which are different from inflammatory symptoms such as redness and swelling, and include itchiness, hot flashes, tingling, and pain that may be caused by the use of the test product.
First, individuals with sensitive skin are selected as test subjects. Then, a small amount of the test product is applied to their cheeks, and at preset intervals their symptoms and level of sensory irritation are recorded. Upon completion of the test, the results are analyzed and assessed.
III. In-use test
In-use tests are conducted to confirm that no physical problems including skin problems are caused by using our products following the instructions for use for a certain period of time.
The subjects answer questionnaires about their skin reactions while using the product. Their answers are analyzed and assessed upon completion of the test.
IV. Patch test for sensitive skin
In order to confirm that even individuals with sensitive skin can safely use our products, patch tests are performed on volunteers with a history of atopic dermatitis or contact dermatitis under 48-hour occlusive patching conditions.
A dermatologist assesses their skin reactions, based on which the irritation index is calculated to make sure that the product is less irritative to the skin.
* Products assessed as less irritative are labeled “TESTED ON SENSITIVE SKIN.”
V. Repeat Insult Patch Test (RIPT)
The RIPT aims to assess the potential of the test product to cause allergic reactions in the skin. A certain amount of the test ingredient or product is applied to the back of the subject, and left for 24 hours under occlusive patching conditions. This is repeated nine times during the course of three weeks, followed by a two-week rest period. After the rest period, another patch is applied to the subject under occlusive conditions, and it is determined whether the subject has allergic reactions to the ingredient or product by examining the subject’s skin condition.
* Products assessed as negative (not causing allergic reactions) are labeled as “DERMATOLOGIST-TESTED.”
VI. Comedogenicity test
The comedogenicity test aims to assess the comedogenic potential of the test product. Comedones are called “menpou” in Japanese.
After applying the test ingredient or product to the back of the subject under occlusive conditions 12 times in a row during the course of four weeks, in the fifth week, samples of the stratum corneum are taken by using a slide glass coated with a peeling agent and examined with a microscope to identify signs of emerging comedones.
* Products assessed as negative (not causing comedones) are labeled “NON-COMEDOGENIC.”
VII. Clinical safety evaluation of eye area products by an ophthalmologist
This test is conducted to assess the safety of our skincare and makeup products used around the eyes. The subject routinely uses the test product following the instructions for use for two weeks. The subject is examined by an ophthalmologist, and takes an ophthalmic exam before and after the test.
* Products for overseas markets that are assessed as negative (safe for application) are labeled “OPHTHALMOLOGIST-TESTED.”
VIII. Human photo maximization test
The human photo maximization test aims to assess the potential of the test product to cause photoallergic reactions. A small amount of the test ingredient or product is applied to the back of the subject, and left for 24 hours under occlusive patching conditions, followed by irradiating the back with light, for a total of six cycles over a two-week period. After a two-week rest period, another occlusive patch is applied to the subject, the subject is again irradiated with light, and it is determined if the subject has photoallergic reactions to the ingredient or product by examining the subject’s skin condition.
Safety Assurance Endpoints
The safety assurance endpoints assessed at Shiseido for cosmetic products are classified into two categories: local toxicity (direct adverse impacts on the part of the body where the product is applied, such as irritation) and systemic toxicity (any systemic adverse effect). Shiseido assures the safety of its products with regard to each assurance endpoint while taking measures to prevent the occurrence of misuse, and protect the environment (ecology). In addition, our safety assurance information is provided to healthcare providers if necessary.
ABOUT US
-
Who we are
-
History
-
Profile
-
Governance
-
Quality Management
-
Supply Network
-
Region/Business
BRANDS
-
Prestige
-
Premium
-
Inner Beauty
-
Life Quality Makeup
SUSTAINABILITY
-
Strategy / Management
-
Society
-
Environment
-
Governance
-
Reports / Data
-
Related Information
INNOVATION
-
Research and Development
-
Research Areas
-
Research outcomes
-
Product safety
-
Product Development Policy
-
Initiatives for doctors and researchers
CAREERS
-
DISCOVER OUR WORLD OF OPPORTUNITY
- Shiseido careers
INVESTORS
-
IR Library